For the first time, neuroscientists have recorded objective biomarkers of chronic pain from inside the brain of individuals suffering from phantom limb pain (PLP) and other forms of chronic pain.
Funded by the National Institutes of Health (NIH) and the Defense Advanced Research Projects Agency (DARPA), the research marks a key advance in mapping chronic pain signals to a specific part of the brain. This long sought-after goal could open the door to new treatments for relieving PLP and other forms of chronic pain.
“There is so much we don’t understand about how pain works,” says lead author Prasad Shirvalkar, associate professor of anesthesia and neurological surgery at the University of California, San Francisco. “By developing better tools to study and potentially affect pain responses in the brain, we hope to provide options to people living with chronic pain conditions.”
Neuropathic pain most commonly occurs due to peripheral nerve damage, but this study focused on individuals whose pain is thought to originate within the brain itself. Participants included individuals with PLP as well as patients suffering from pain disorders resulting from strokes.
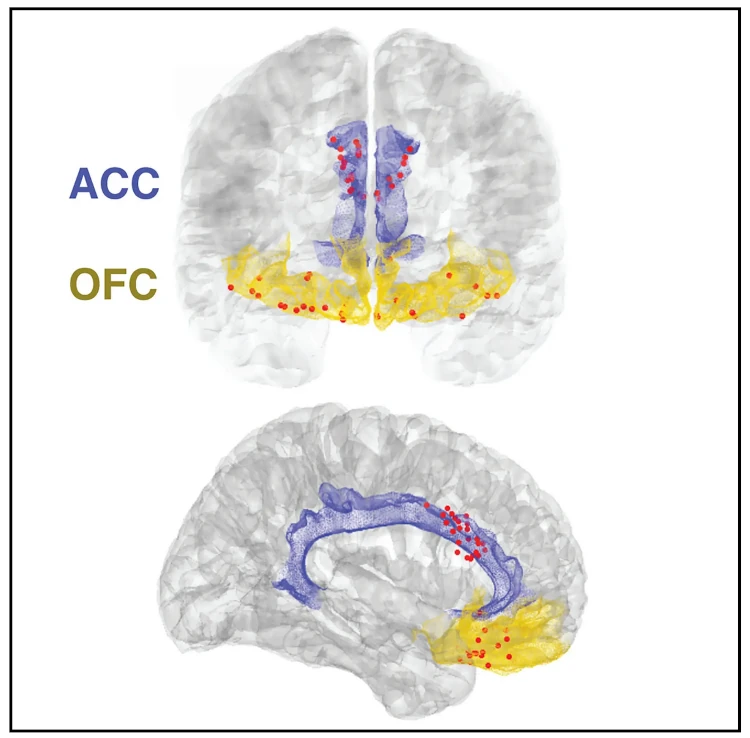
Traditionally, researchers study chronic pain by using questionnaires to gather patient-reported data about pain intensity, frequency, and emotional impact. This study correlated self-reported information with objective measurements of neurological activity in two key areas of the brain: the anterior cingulate cortex (ACC) and the orbitofrontal cortex (OFC).
“Functional MRI studies show that the ACC and OFC regions of the brain light up during acute pain experiments,” Shirvalker explains. “We were interested to see whether these regions also played a role in how the brain processes chronic pain. We were most interested in questions like how pain changes over time, and what brain signals might correspond to or predict high levels of chronic pain.”
To correlate episodes of chronic pain with corresponding brain signals, the researchers surgically implanted electrodes targeting the study subjects’ ACC and OFC regions. Several times a day, each participant was asked to record the intensity, type, and emotional impact of their pain. They would then click a remote-control device to take a snapshot of the activity in their ACC and OFC at that exact moment.
Using machine-learning analyses, the research team was eventually able to predict each participant’s pain state based on the activity in the OFC. Ongoing and future work involving more participants will be necessary to determine whether different pain conditions share the OFC activity seen in these patients and how the signatures differ among persons with different pain conditions.
Identifying specific biomarkers will constitute a major step toward developing personalized pain management for individuals with PLP and establishing therapies that can alter brain activity to relieve chronic pain. “We are hopeful that building from these preliminary findings could lead to effective, non-addictive pain treatments,” says Walter Koroshetz, director of the National Institute of Neurological Disorders and Stroke (part of NIH).
The full study appears in the June 2023 edition of Nature Neuroscience.




