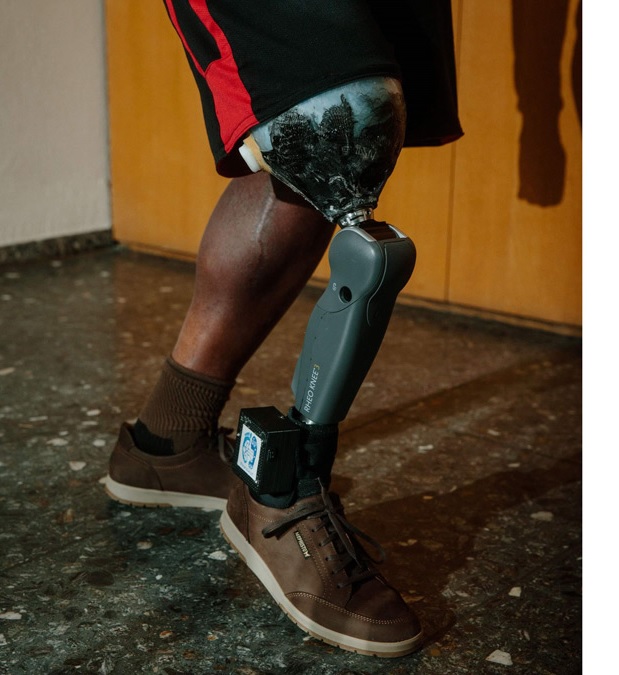
When we first took note of SensArs Neuroprosthetics’ pioneering bionic leg, the SENSY, about two years ago, we described it as having the potential to “merge” with amputees’ bodies. Earlier this year, we explained that the device “not only hears the brain’s commands, it also sends information back . . . . enabling users to ‘feel’ through the prosthesis.”
If you’ve been wondering whether this hot-shot technology would ever advance from the testing lab into the marketplace, there’s good news: The SENSY has just received Breakthrough Device Designation from the US Food and Drug Administration. This interim seal of approval expedites the SENSY’s path to commercial viability, giving it a clear shot to become the first widely available “sensing” leg.
“We tried to design a device that would enable intuitive contact with the residual nervous system,” said Stanisa Rapopovic, one of the SENSY’s chief developers, in a recent interview with Technology Networks. “[Patients] don’t want a device that they have to train to use. They want to use it intuitively.”
The SENSY achieves that outcome via sensors that are embedded in the foot (and knee, for above-knee devices), then connected directly to nerves in the residual limb. The resulting system produces naturalistic sensations of force, pressure, and position, giving wearers a type of neurological feedback that resembles incoming signals from an anatomical limb. In clinical trials conducted in 2019 and 2020, the SENSY enabled amputees to walk with less effort, more confidence, and better balance than a conventional prosthesis. In another experiment, subjects were able to demonstrate a deft touch on an automobile gas pedal, using feedback from the prosthesis to apply finely calibrated intervals of pressure on the accelerator.
Rapopovic, who won this year’s prestigious Science & PINS Prize from the American Association for the Advancement of Science, says the SENSY’s impact on qualify of life goes beyond the execution of everyday tasks. With a direct feedback loop between brain and bionic limb, test subjects reported the SENSY used up far less mental energy than a traditional device. And because it involves direct stimulation of peripheral nerves in the residual limb, the system tends to blunt the effect of phantom limb pain.
“The neuromodulation system triggered by lower limb prosthesis developed by Dr. Raspopovic and colleagues can allow more realistic sensations at lower metabolic cost and improved awareness of one’s own actions, potentially improving quality of life for millions of individuals with amputations,” wrote the contest’s judges.
The FDA’s Breakthrough Designation puts the SENSY on a fast track to eligibility for Medicare reimbursement, which in turn makes the device more attractive to investors and potential clinical partners. Stay turned for updates about where, when, and how the SENSY is available on a trial basis in the US.